What Parents Need to Know About the Introduction of the 6-In-1 Vaccine Under the National Immunisation Program for Infants and Children
Pathogenic microorganisms, including bacteria, viruses, parasites or fungi are the causes of infectious diseases. These diseases can spread to humans through a number of ways, such as person-to-person through direct touch, water or foodborne, or through the spraying of infectious particles into the atmosphere, and through insects such as mosquitoes.
The National Immunisation Program (NIP) is a government initiative to curb the spread of infectious diseases. The Ministry of Health of Malaysia (MOH) introduced the NIP in the 1950s by providing free vaccination services to children in Malaysia.
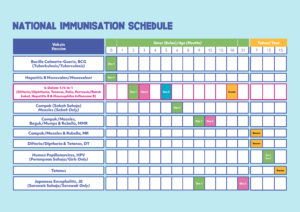
Currently, the NIP protects children against twelve (12) vaccine-preventable diseases i.e. tuberculosis, Hepatitis B, diphtheria, tetanus, whooping cough (pertussis), polio, Haemophilus influenzae type b, measles, mumps, rubella, Japanese encephalitis (JE), and cancer caused by the human papilloma virus (HPV). Children receive all these vaccines within the first 2 years of life, except for the HPV vaccine which is given to teenage girls at 13 years of age. Booster doses for some vaccines are also given during school age.
Since the introduction of the NIP, improvements have been made from time to time, including the introduction of the Hepatitis B vaccine in 1989, the Haemophilus influenzae type b (Hib) vaccine and Measles-Mumps-Rubella (MMR) in 2002 and the 5-in-1 combination vaccine in 2008, to ensure its implementation is in line with the developments in science and vaccine technology as well as taking into consideration the recommendation from World Health Organisation (WHO). This is important in ensuring that immunisation can provide a complete and optimal protection for children in Malaysia.
This year, the Ministry of Health Malaysia (MOH) once again made improvements to the NIP by introducing a 6-in-1 vaccine and announcing a new immunisation schedule.
The Director General of Health, Tan Sri Dato’ Seri Dr Noor Hisham Abdullah, issued a statement recently regarding the introduction of this vaccine and the change to the new immunisation schedule for children who get their immunisation at MOH Health Clinics. He said, “The switch in the immunisation schedule is done following the change in the type of vaccine used, from the 5-in-1 vaccine to 6-in-1 vaccine, under the National Immunisation Program for children. The implementation will be conducted in stages, which will start as early as November 2020 depending on the availability of this vaccine supply at MOH health facilities.”
The Benefits of the ‘6-In-1’ Vaccine
Dr Jamiatul Aida Md Sani, Senior Principal Assistant Director at the Ministry of Health’s Disease Control Division, said the move to introduce the 6-in-1 vaccine in the NIP was made with careful consideration by the National Immunisation Policy and Practice Committee.
She explained that the use of the 6-in-1 vaccine in the National Immunisation Program has been adopted by 49 countries in the world. Therefore, the 6-in-1 vaccine exchange is a good step forward in line with developments in other developed countries.
She explained that previously, the 5-in-1 vaccine that prevented diphtheria, tetanus, pertussis, polio and Haemophilus influenzae type b (Hib) were given to each child aged 2, 3, 5 and 18 months in 4 doses. Meanwhile, the Hepatitis B vaccine was given separately in 3 doses to each child after birth and at the age of 1 and 6 months. This means that 7 injections were needed to prevent these 6 diseases.
She added, “Switching to the 6-in-1 vaccine prevents diphtheria, tetanus, pertussis, polio, Haemophilus influenzaetype b (Hib) and Hepatitis B benefits children, of which only 4 doses of vaccine are required for each child (at 2, 3, 5 and 18 months). Meanwhile, a separate Hepatitis B vaccine should only be given as one injection after birth. This means that the number of injections is reduced to 5 injections to prevent 6 diseases.”
| The Six (6) Diseases Prevented By ‘6-In-1’ Vaccine | |
| Diphtheria | Infection of the throat and nose that can cause breathing difficulties, heart failure, paralysis and death. |
| Tetanus | Infection of the nervous system that can cause muscle cramps especially in the jaw and neck and which often cause death. |
| Pertusis (Whooping cough) | An infection of the respiratory tract that can cause pneumonia, seizures, brain inflammation and can also cause death. |
| Poliomyelitis | Infection of the nervous system that can cause permanent paralysis and can be fatal. |
| Hepatitis B | An infection of the liver that can cause severe liver damage and lead to liver cancer. |
| Haemophilus influenzae type b | An infection that cause inflammation of the lining of the brain, bacterial infections in the blood and pneumonia and which can be fatal. |
Dr Rozita Binti Ab Rahman, Senior Principal Assistant Director from the immunisation unit, Family Health Development Division, Ministry of Health Malaysia, said “There are several advantages in the use of this 6-in-1 vaccine. The main advantage is the reduction of the number of injections required and immunisation visits at Health Clinics.”
“The second advantage is from the point of view of the comfort of the baby itself. Injections are painful for babies, even for a moment. Less injections means less episodes of pain and crying. Thirdly, healthcare personnel will have more time during clinic appointments to conduct assessments of the babies’ health and development such as their growth and their sensory and intellectual development.”
The 6-in-1 Vaccine is Safe for Children
Ms Norleen bt Mohamad Ali, Senior Principal Assistant Director, Pharmacovigilance Section at the Centre of Compliance & Quality Control, National Pharmaceutical Regulatory Agency (NPRA), also shared her views on the safety of the 6-in-1 vaccine.
She said, “The 6-in-1 vaccine is safe to use. Just like other vaccines, this vaccine will go through a few clinical trial phases including a large-scale trial before it can be used commercially. This is to ensure the effectiveness and safety of the vaccine to the recipients.”
“In Malaysia, a vaccine goes through a strict and thorough registration process by the Drug Control Authority (DCA) under the Ministry of Health. After the approval for registration is granted, only then can the vaccine be used and marketed in Malaysia, including being introduced in the National Immunisation Programme.”
Ms Norleen continued, “Hexavalent vaccine is not new in Malaysia. As of October 2020, there are two 6-in-1 vaccine products registered in Malaysia and the usage is increasing every year. Both vaccines have been used in private health facilities in 2006 and 2013, respectively.”
Ms Norleen also stated, “In Malaysia, the majority of adverse effects following immunisation or also known as AEFI, reported for this vaccine is mild and can be resolved within a few days, with or without treatment. The pattern of the reported AEFI are similar with other vaccines. However, the serious AEFI still can occur but the frequency is rare. Parents are encouraged to report the AEFI experienced by their babies, even if it is a mild reaction, such as fever, swelling or itching at the injection site.”
“The NPRA constantly and actively monitors all the vaccines in Malaysia including the 6-in-1 vaccine. AEFI, either mild or serious, needs to be reported.” She stated that parents can report the AEFI through healthcare providers or directly to the NPRA via the NPRA website at www.npra.gov.my by clicking on the “Consumer” tab and selecting “ConSERF Online Reporting”.
Ms Norleen continues to convince parents that the 6-in-1 vaccine is safe. She concluded, “The benefit of the vaccination to prevent the disease is higher compared to the risk of AEFI which is mostly mild and harmless.”
 Views of a Pediatrician
Views of a Pediatrician
Immunise4Life programme chairman and Consultant Paediatrician and Paediatric Cardiologist, Datuk Dr Zulkifli Ismail, welcomed the changes made by the MOH in introducing the 6-in-1 vaccine in the new immunisation schedule. He said, “The MOH’s decision to give this vaccine to babies and children in Malaysia is a good thing.”
He also shared his experience of using this vaccine in private health facilities since it was approved in Malaysia. “I have been giving this vaccine to babies and children in private health facilities for many years.”
According to him, throughout his experience giving this vaccine to babies, no adverse effects have been reported by parents. Babies may cry for a while after receiving a vaccine injection, but this is normal. They will stop crying after being persuaded or breastfed and this is not something to worry about.
He added, “Other mild side effects include irritation, pain and redness at the injection site. Most babies do not show any reaction at all.”
Dr Rozita, Dr Aida, Ms Norleen and Datuk Dr Zulkifli all agree that the use of the 6-in-1 vaccine in the new immunisation schedule is a good decision. They see the use of this 6-in-1 vaccine as beneficial to parents and children in Malaysia.
This article is courtesy of the Immunise4Life programme by Ministry of Health Malaysia, Malaysian Paediatric Association and Malaysian Society of Infectious Diseases & Chemotherapy, supported by the Vaccination is Protection for Kids initiative. For more information, visit www.ifl.my
