
Source: www.theplaidzebra.com
“Which antibiotic should I use?” It’s a question we ask ourselves when we want to initiate antibiotic treatment for our patients. When patients approach us at the clinic or hospital with fever or other symptoms of infection, they hope that they will be treated correctly. That includes prescribing the appropriate antibiotic. Sometimes, patients request for the most elaborate broad-spectrum antibiotics. But do we need to eliminate as many pathogens as possible? The answer is definitely not.
There is a recent increase of Methicilln-resistant Staphylococcus aureus (MRSA), Extended spectrum beta-lactamase (ESBL) and Carbapenem-resistant enterobacteriaciae (CRE). We are actually the culprit; we – the medical personnel who keep using broad-spectrum antibiotics that result in antibiotic resistance. Are we aware that our antibiotic options are limited? Very soon we will succumb to a situation in which there are no more antibiotics to treat these increasingly resistant bacteria if we continue to prescribe antibiotics wantonly.
In order to prescribe the appropriate antibiotics, we need some basic knowledge on common bacteria causing common infections. Once we identify and update our knowledge on possible target organisms, the golden rule is to use the narrowest spectrum antibiotic that retains appropriate organism susceptibility.
Let’s start with common upper respiratory tract infections (URTI). The aetiological bacteria are commonly Staphylococcus and Streptococcus species, which are adequately treated with the penicillin group of antibiotics. It is appropriate to use oral penicillin V, ampinopenicillins (ampicillin) or even 1st generation cephalosporins like cephalexin. Common bacterial causes of community-acquired pneumonia include those that cause URTI, as well as beta lactamase groups like gram-negative bacteria (Enterbacteriaciae species, Klebsiella pneumonia and Haemophilus influenza). An ampinopenicillin group cannot cover beta lactamase infections. Therefore, beta-lactamase resistant penicillins like augmentin (amoxicillin and clavulanic acid) and second-generation cephalosporin such as cefuroxime, can be used. If there is a need to cover for atypical organisms, macrolides such as erythromycin or azithromycin can be given.
Skin infections such as cellulitis are mainly caused by Staphylococcus and Streptococcus species. Group A Streptococcus like Streptococcus pyogenes may cause purulent cellulitis or blisters. In general, beta-lactamase resistance penicillins are the antibiotics of choice, eg. cloxacillin, augmentin, and Unasyn (ampicillin and sulbactam). Both augmentin and Unasyn can target both gram positive and gram negative bacteria. Do bear in mind that gram-negative bacteria can be the aetiological organism in immunocompromised individuals or refractory patients.
Leg ulcers can be caused by both gram negative and gram positive bacteria, therefore augmentin and Unasyn may be used. However, Pseudomonas aeruginosa could be the aetiological agent in patients with poorly-controlled diabetes, intravenous drug abusers or immunodeficient patients who present with chronic, deep and dirty leg ulcers. Good clinical judgment must be used in cases where pseudomonas-covering antibiotics – 3rd and 4th generation cephalosporins (ceftazidime, cefepime), aminoglycosides or carbapenems (imipenem) – are considered. Don’t forget the role of wound debridement and appropriate dressings as well.
Urinary tract infections (UTI) are commonly caused by Escherichia coli and Klebsiella species bacteria, which are beta-lactamase Enterobacteriaciae. Other causative organisms include Staphylococcus saprophyticus. The selection of antibiotics includes 1st and 2nd generation cephalosporins (cefuroxime and cephalexin), beta-lactamase resistance penicillin (augmentin) and also bactrim (trimetoprim-sulphametoxazole). In complicated UTI or suspected Pseudomonas infections, flouroquinolones like ciprofloxacin can be prescribed.
Community-acquired sepsis, in patients without co-morbidities, is often caused by Staphylococcus, Streptococcus and Enteriobacteriaciae. Antibiotics like augmentin, doxycycline and 2nd generation cephalosporins are the appropriate choice. Avoid jumping into 3rd generation cephalosporins like ceftriaxone as empirical treatment for these patients to prevent the emergence of antibiotic-resistance organisms like MRSA. Meliodosis must be suspected in patients with multiple underlying medical issues. Ceftazidime and carbapenems can be considered in high-risk patients with chronic renal disease, diabetes mellitus or thalassaemia when they present with sepsis.
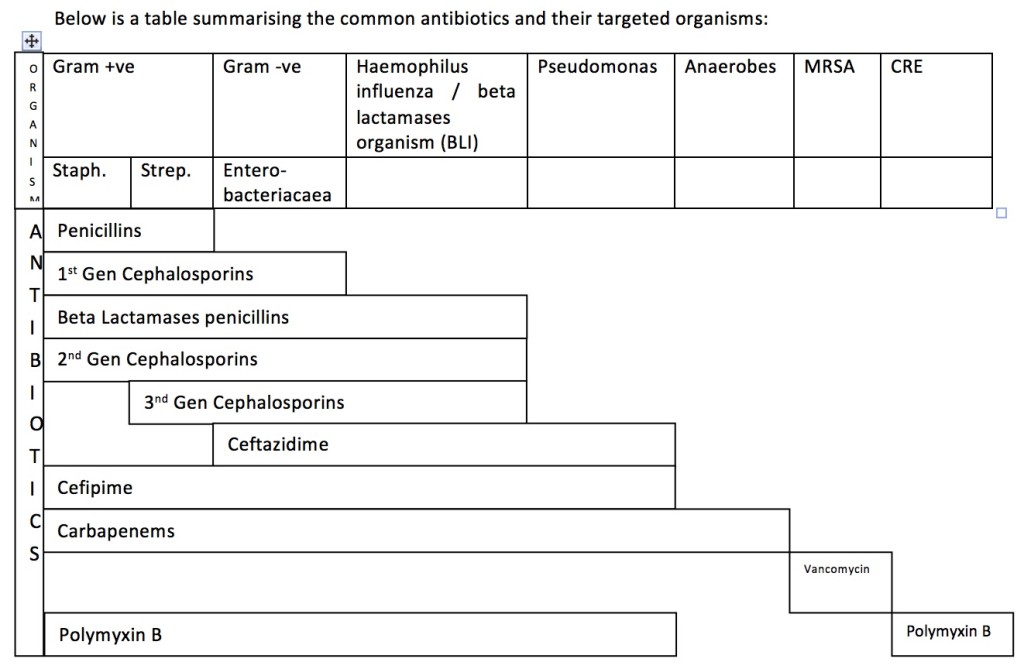
In most cases, 1st line antibiotics in any of the infections above do not cover Pseudomonas infections. Pseudomonas infection usually occurs in immunocompromised patients; people who work with contaminated soil, water or animals (farmers, labourers); patients with haematological diseases; patients undergoing chemotherapy; intravenous drug abusers; diabetic patients; the elderly; post-surgery patients; those on continuous urinary catheterisation or other instruments; and patients in the intensive care setting. The decision to start antibiotics that cover Pseudomonas can only be made after considering the patient’s clinical presentation and potential risks. Antibiotics to be considered in those cases include aminoglycosides (gentamicin), 3rd generation cephalosporins (ceftazidime), 4th generation cephalosporins (cefepime), flouroquinolones (ciprofloxacin) and carbapenems (meropenem or imipenem). Carbapenems are generally reserved for serious infections caused by organisms resistant to other beta-lactam antibiotics or in patients with renal disease that are at risk of aminoglucoside-related nephrotoxicity.
Overall, in any case of infection that requires in-patient care and antibiotics, the antibiotics need to be deescalated based on blood culture and sensitivity results. Vancomycin and linezolid should be kept aside for MRSA infections only, while polymyxin should be reserved for CRE infections.
There are other precautions we can take to avoid emergence of antibiotic resistant organism besides using antibiotics wisely. They include proper hand hygiene practices; discharging patients only after they have received appropriate treatment and are clinically improving; and adequate isolation of patients infected with antibiotic-resistant organisms. Hopefully this serves as a reminder, for not only medical practitioners but also junior medical doctors and students, to think before prescribing antibiotics. Let us prescribe antibiotics wisely!
This article was written by Dr Shaiful Ehsan.
Dr Mohd Shaiful Ehsan is a family medicine master trainee in the International Islamic University Malaysia.
References:
- National Antibiotic Guidelines 2014
- American Family Physician – Appropriate Prescribing of Oral beta lactam antibiotics
- Johor Medical Updates 2015 – Topic 4: Prescribing antibiotics – an update on latest guideliens (5th September 2015)
[This article belongs to The Malaysian Medical Gazette. Any republication (online or offline) without written permission from The Malaysian Medical Gazette is prohibited.]
